Hydrogen is the smallest, most basic atomic element, sitting at the very top left of the periodic chart. It’s abundant, ubiquitous, powerful, and clean and can potentially solve some of the world’s energy problems. Like every other form of energy, there are pros, cons, benefits, and challenges.
This post, which focuses on hydrogen production, is the first of a three-part series leading up to a related webinar scheduled for May 26, 2022. Later I’ll expand further down the Hydrogen Value Chain, which is incredibly diverse. There are a myriad of possible end uses that make hydrogen a key player in “sector coupling” – a common resource used by many industries. I’ll dedicate a later post looking at a few scenarios specifically involving power generation.
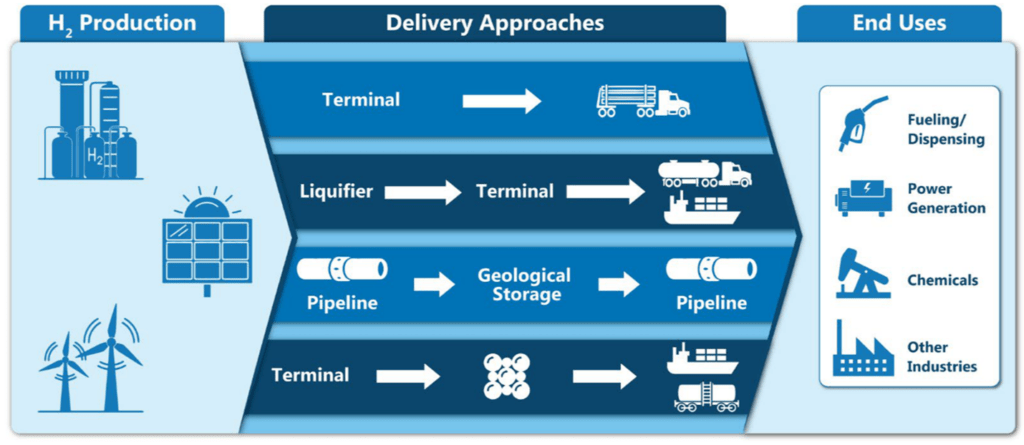
Source: U.S. Department of Energy
Making Hydrogen
Hydrogen is too small to live alone. It’s always found attached to other molecules in a compound. Its two most common partners are oxygen, as in water (H2O) and carbon, as in natural gas (CH4). Before using hydrogen as a fuel, we must first isolate it, which requires work. How that work is done and which compound it comes from matters greatly in terms of cost and environmental impact. I’ll explain the most common methods: steam methane reforming (SMR) and electrolysis.
Steam Methane Reforming (SMR) – Hydrogen From Natural Gas
The largest component of natural gas is methane, a compound with one carbon atom and four hydrogen atoms (CH4). As the name implies, SMR uses steam to break apart methane into hydrogen, carbon monoxide, and carbon dioxide. This process is historically the cheapest and most common source of hydrogen. We call hydrogen taken from fossil fuels “grey” hydrogen. By using carbon capture, utilization, and storage (CCUS), we can promote it to “blue” hydrogen and take a step toward the U.S. government’s net zero goal. However, it still depends on natural gas availability and emits CO2 that needs to be contained. This is not the droid we’re looking for.
Electrolysis – Hydrogen from Water (H2O)
Electrolysis uses electricity directly to separate hydrogen from oxygen in water. An electrolyzer can use any source of electricity, but hydrogen is considered “green” when it’s produced using a carbon-free power source such as wind, solar, or nuclear (learn more about the Colors of Hydrogen in this blog post). Electrolysis is more expensive than SMR but is becoming more affordable as technology evolves and the costs of renewable power decrease. That trend line is very favorable. Wind and solar capital costs have dropped significantly in the past few years.
There are several types of electrolyzers, each with different costs, efficiencies, and suitable use cases. The two most common are Alkaline and Proton Exchange Membranes (PEM). Alkaline is the most mature and cost-effective, whereas PEM has faster response and simpler maintenance but costs more.
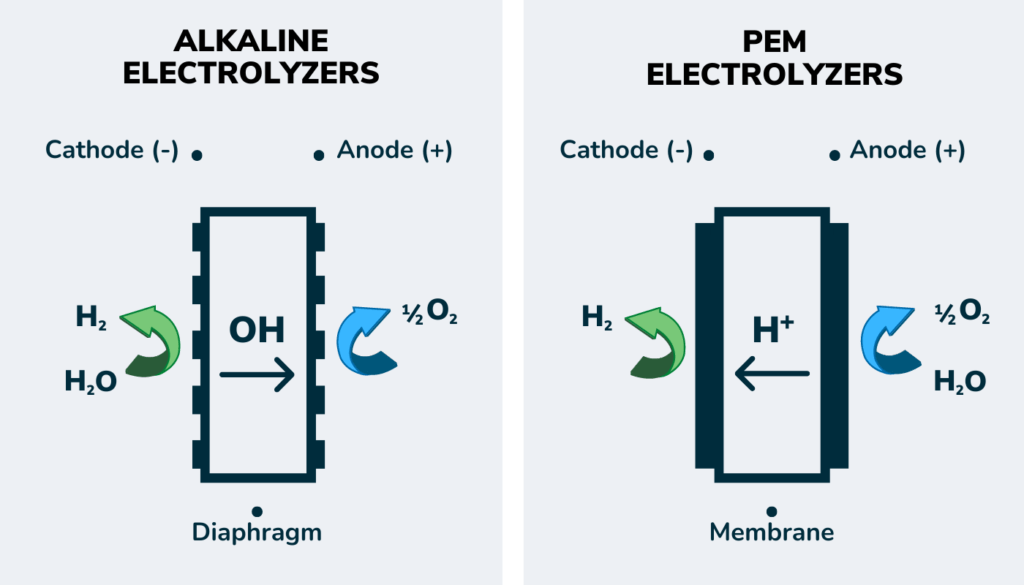
You can learn more about electrolyzers in this U.S. Department of Energy fact sheet.
Despite the additional cost for electrolysis compared to SMR, electrolyzers bring some significant benefits:
- An electrolyzer can use cheap or negatively priced power from renewables, making it cost-effective. Rather than curtailing nuclear, wind, or solar when demand is low, excess MWh can be stored in the form of hydrogen to produce power later.
- Electrolyzers are great demand-side management (DSM) resources to help flatten the infamous “duck curve.”
- They can ramp fast enough to provide regulation and other grid services like batteries.
- Unlike SMR, an electrolyzer is not prone to natural gas price shocks or supply threats from hostile governments. Current events in Europe underscore the importance of energy security and supply diversity.
- Electrolyzer hydrogen is green, which is ultimately more climate-friendly than blue.
Hydrogen Storage
Clearly, one of hydrogen’s advantages is gravimetric density (energy per unit of mass), which is greater than most other fuels. A kilogram of hydrogen has about three times as much energy as a comparable amount of diesel or gasoline. However, it also has a very low volumetric density, so it needs to be compressed for efficient storage – another energy-intensive process. Hydrogen liquefication requires very high pressure and cryogenic temperatures, which are serious challenges for containment and transportation. Big money is going into addressing those challenges.
Source: U.S. Department of Energy
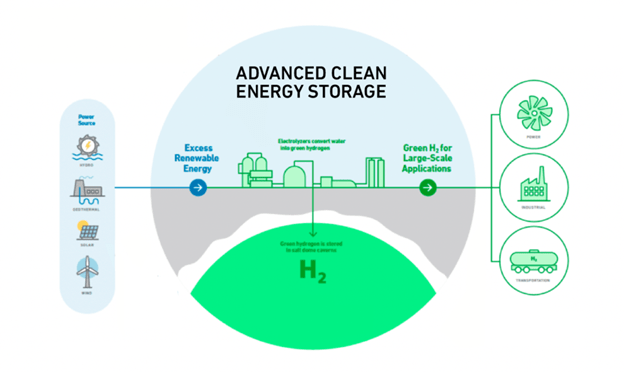
A viable storage solution for natural gas also works for hydrogen – giant salt caverns. One project getting much attention is the ACES Delta project in Utah, where they expect to produce 10 to over 100 tons of green hydrogen per day using electrolysis banks from 20 to 200 MW. This Advanced Clean Energy Storage (ACES) hub will be the country’s largest hydrogen gas and storage hub, initially storing over 300 gigawatt-hours of clean energy to serve multiple industries, including power generation and transportation.
Hydrogen to Power
Once we have produced and stored hydrogen, several options can convert it back into electricity. Like the production side, the alternatives for using hydrogen to generate electricity power span the carbon rainbow. The transition to a green hydrogen economy will be gradual, and generation solutions will be driven by NPV based on specific use cases.
Examining those options is the focus of my next blog post.
We’ve hosted an educational webinar, “Hydrogen Power: Opportunities & Challenges.” If you missed it, request the slides! If you’re interested in what PCI can do for you, check out our hydrogen solution.







